Order of a Reaction
Order of a Reaction: Overview
In this topic, we will learn to calculate order of a reaction. It also deals with the concept of molecularity. We will also learn the difference between the order of a reaction and its molecularity with the help of examples.
Important Questions on Order of a Reaction
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
For a reaction, , the rate is given by , hence, the order of the reaction is:
Order of a reaction is the sum of exponents of _____ in the rate equation.
Order of a reaction with rate constant is :
Rate constant and rate of a reaction have the same units for reactions of _____ order.
What is the order for the following reactions?
, rate
The rate law for the gas-phase reaction is rate What is the order of the reaction with respect to each of the reactants and what is the overall order of the reaction?
Explain the term order of a reaction with examples.
The following results have been obtained during the kinetic studies of the reaction:
| Experiment | Initial rate of formation of | ||
| I | |||
| II | |||
| III | |||
| IV |
Determine the rate law and the rate constant for the reaction.
In a reaction between and , the initial rate of reaction was measured for different initial concentration of and as given below:
What is the order of the reaction with respect to and ?
Suppose in a chemical reaction , it is found that the rate of the reaction doubles when the concentration of is increased four times. The order of the reaction with respect to is
In a given reaction condition N2O5 decomposes as
i)
and same product competes to form N2O3
ii)
If initially 1 mole of N2O5 was introduced into a one litre flask subjected to reaction conditions molar concentration of N2O at equilibrium was found to be 0.2 M. Determine Kc for second reaction.
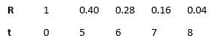
Concentration of reactant w.r.t. time varies as
The order of reaction is:
For a reaction , rate . What is (i) molecularity and (ii) order of reaction ?
What is the order of the reaction which has a rate expression,
For the reaction, products, when the concentrations of and both were doubled, the rate of the reaction increased from to . When the concentration of alone is doubled, the rate increased from to .
Which one of the following statements is correct?
For the reaction, the order of reaction with respect to is
A hypothetical reaction,
follows the following mechanism
........fast
......slow
.......fast
The order of the overall reaction is
For a chemical reaction the rate of reaction increases by a factor 1.837 when the concentration of X is increased by 1.5 times. The order of the reaction with respect to X is
What is the order of a reaction which has a rate expression